For an elementary reaction the temperature dependence of the rate constant is given by the Arrhenius equationK(T) =νe^(-Ea/RT) where ν is called the preexponential factor (sometimes called prefactor) and Ea the activation energy (in kJ mol^(–1)).
来源:学生作业帮助网 编辑:作业帮 时间:2024/11/29 01:48:55
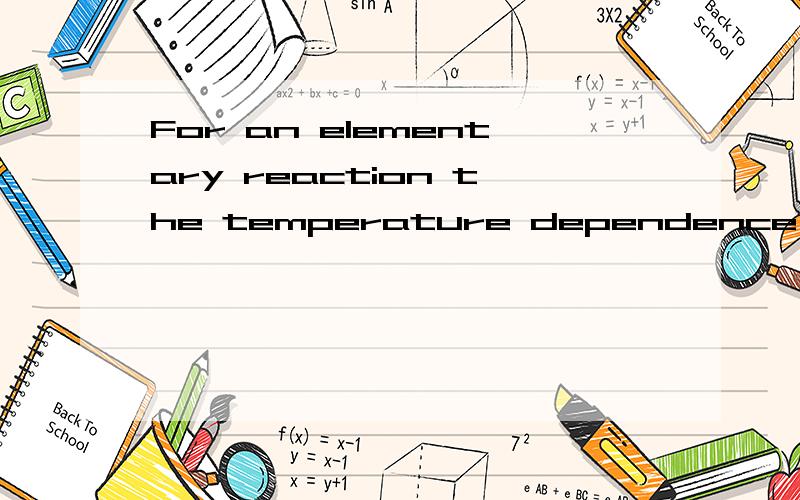
For an elementary reaction the temperature dependence of the rate constant is given by the Arrhenius equationK(T) =νe^(-Ea/RT) where ν is called the preexponential factor (sometimes called prefactor) and Ea the activation energy (in kJ mol^(–1)).
For an elementary reaction the temperature dependence of the rate constant is given by the Arrhenius equation
K(T) =νe^(-Ea/RT)
where ν is called the preexponential factor (sometimes called prefactor) and Ea the activation energy (in kJ mol^(–1)).One can also express the activation energy in joules per single molecule (a very small number),provided one replaces the gas constant,R,by Boltzmann constant,k_B.
我想知道气体常数是怎么用Boltzmann常数来取代的
For an elementary reaction the temperature dependence of the rate constant is given by the Arrhenius equationK(T) =νe^(-Ea/RT) where ν is called the preexponential factor (sometimes called prefactor) and Ea the activation energy (in kJ mol^(–1)).
如果我们用Boltzmann constant来表示Universal gas constant R的话,activation energy 的单位就是joules per single molecule了.(一般非常小)
气体常数比Avogadro's constant就是 Boltzmann constant.(这一点你从dimensional analysis的角度很好理解,看看单位的变化)