glucose can be regarded as a simple molecular solid of formular HOCH2CH(OH)CH(OH)CH(OH)CH(OH)CHO it isreadily soluble in water because water molecules form hydrogen bonds to1.the carbon atoms of the glucose molecules2.the oxygen atom of the C=O group
来源:学生作业帮助网 编辑:作业帮 时间:2024/11/18 16:22:06
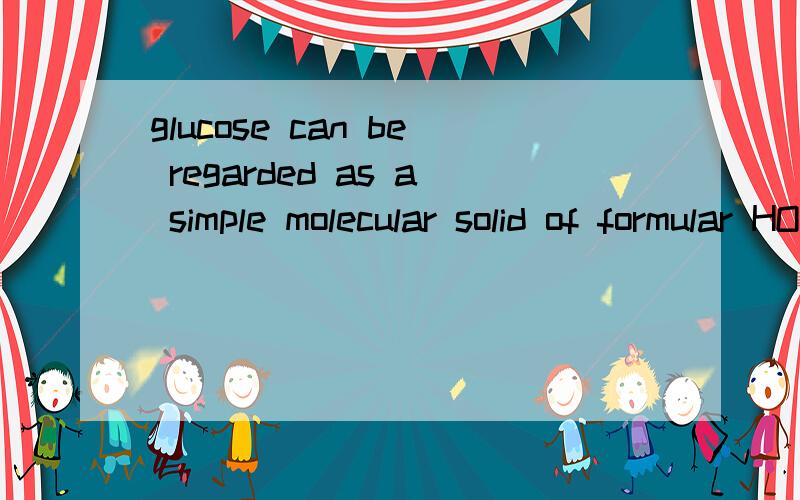
glucose can be regarded as a simple molecular solid of formular HOCH2CH(OH)CH(OH)CH(OH)CH(OH)CHO it isreadily soluble in water because water molecules form hydrogen bonds to1.the carbon atoms of the glucose molecules2.the oxygen atom of the C=O group
glucose can be regarded as a simple molecular solid of formular
HOCH2CH(OH)CH(OH)CH(OH)CH(OH)CHO
it isreadily soluble in water because water molecules form hydrogen bonds to
1.the carbon atoms of the glucose molecules
2.the oxygen atom of the C=O group of the glucose molecules
3.the OH groups of the glucose molecules
glucose can be regarded as a simple molecular solid of formular HOCH2CH(OH)CH(OH)CH(OH)CH(OH)CHO it isreadily soluble in water because water molecules form hydrogen bonds to1.the carbon atoms of the glucose molecules2.the oxygen atom of the C=O group
问葡萄糖的水溶性大 是为什么
我们知道是氢键,显然是水分子跟羟基结合形成氢键
答案3
选3啊,题目问的是葡萄糖易溶于水的原因是跟哪个基团形成氢键,那自然是跟羟基形成氢键了